Introduction:
In the rapidly advancing field of medicine, researchers are increasingly intrigued by proteins and peptides. As vital signaling molecules and potential drug candidates, the synthesis and customization of peptides have become pivotal. This article delves into the realm of custom peptide library synthesis, analyzing its significance in medical research and the latest advancements.
Custom Peptide Library Design and Construction:
The design and construction of peptide libraries are critical steps in custom peptide library synthesis. This section explores how to design libraries based on research needs, selecting suitable synthesis strategies and technologies. Topics covered include peptide sequence design, method selection, and quality control measures such as purity and mass spectrometry.
Applications of Custom Peptide Libraries in Drug Development:
Custom peptide library synthesis plays a crucial role in drug development. This section extensively discusses the use of custom peptide libraries in identifying potential drug targets, screening drug candidates, and enhancing drug specificity and efficacy. Through case studies, it illustrates successful experiences and future directions in drug development utilizing custom peptide libraries.
Technological Innovations and Trends:
Peptide synthesis technology continually undergoes innovation and development. This section tracks the latest technological trends, including but not limited to solid-phase synthesis, liquid-phase synthesis, and genetic engineering techniques. By evaluating new technologies, it provides researchers with insights to choose the most suitable peptide synthesis method for their research purposes.
Applications of Custom Peptide Libraries in Disease Research:
Beyond drug development, custom peptide libraries play a crucial role in disease research. This section delves into the applications of custom peptide libraries in areas such as cancer, neurological disorders, immune system diseases, presenting case studies and revealing new biological mechanisms discovered through custom peptide library research.
Challenges and Future Outlook:
Despite significant achievements in custom peptide library synthesis, challenges persist. This section discusses current technical, economic, and ethical challenges, offering insights into potential future developments. It explores avenues such as automation, big data analysis, and personalized medicine applications.
Conclusion:
Through a comprehensive analysis of custom peptide library synthesis, this article underscores its significance in medical research and drug development. It serves as a guide for researchers, providing an in-depth understanding and application framework for custom peptide library synthesis technology.
By dissecting the field of custom peptide library synthesis, this article aims to offer a thorough understanding and application guide, fostering continuous innovation and development in this critical domain.


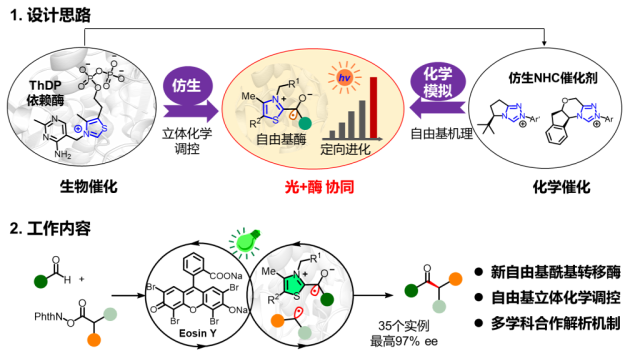
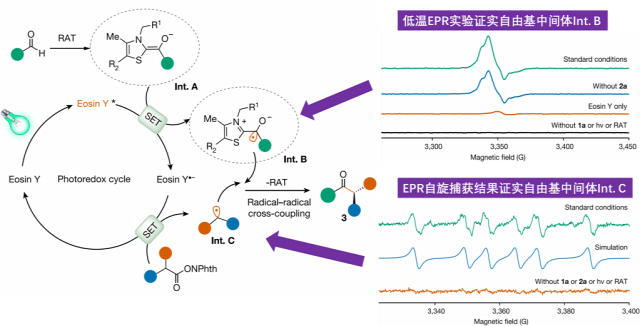
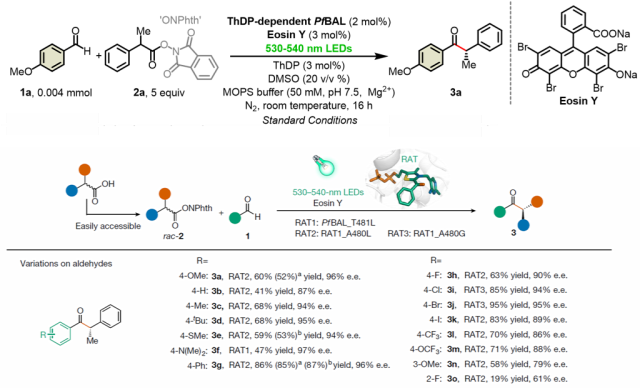 For the photoenzyme dual catalytic system, Professor Tian Changlin's team applied low-temperature (80K) electron paramagnetic resonance (EPR) experiments, capturing the ThDP-derived ketyl free radical (Int. B). Through EPR spin trapping experiments, they detected characteristic six-line splitting spectra in the standard reaction system, confirming it as an intermediate benzylic radical (Int. C) and the free radical product after addition with the capture agent. This provided direct evidence for unraveling the key to the new enzyme reactivity and the source of high stereochemical selectivity.
For the photoenzyme dual catalytic system, Professor Tian Changlin's team applied low-temperature (80K) electron paramagnetic resonance (EPR) experiments, capturing the ThDP-derived ketyl free radical (Int. B). Through EPR spin trapping experiments, they detected characteristic six-line splitting spectra in the standard reaction system, confirming it as an intermediate benzylic radical (Int. C) and the free radical product after addition with the capture agent. This provided direct evidence for unraveling the key to the new enzyme reactivity and the source of high stereochemical selectivity.