In contrast to proteins and small molecules, peptides represent a unique class of pharmaceutical compounds that are biochemically and therapeutically distinct from both. As intrinsic signaling molecules for many physiological functions, peptides offer an opportunity for therapeutic intervention that closely mimics natural pathways. In recent years, peptides have received increasing attention as a therapeutic approach.
The origin and development of in vitro peptide drugs
Polypeptides are amino acid derivative compounds containing at least one amide (peptide) bond. From a structural point of view, polypeptides include various types of peptides, such as linear peptides, cyclic peptides, delipidated peptides, etc. According to function, they can also be divided into antibacterial peptides and hormones. Regulatory peptides, neuroactive peptides, etc. [1].
In the early 20th century, research on peptides focused primarily on the effects of human signaling hormones. Insulin is a classic example of endogenous hormone therapy. It was the first peptide drug to be used clinically and is by far the most commercially successful [2] because it revolutionized the treatment of type I diabetes.
Despite the success of early hormone analogs, the production of longer peptides was limited by synthetic methods. Therefore, selective expression of endogenous human peptides and proteins in cell culture systems is highly desirable, and the emergence of recombinant technology is a milestone in the development of peptide drugs. In 1982, the first recombinantly produced human peptide, somatostatin, was produced. Subsequently, the booming genetic engineering has realized the adjustment of individual amino acids to improve the absorption, distribution, metabolism and excretion characteristics of peptides in the body. Display technologies such as phage display, as well as new chemical methods are also driving the development of this field [2].
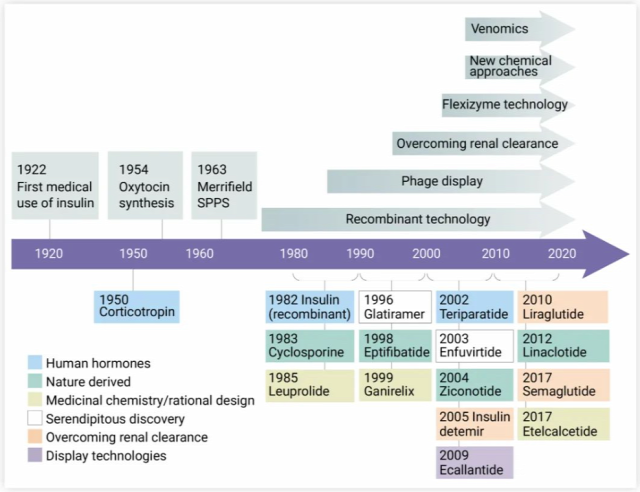
Figure 1. Development timeline of peptide drugs[2]
Advantages, disadvantages and new attempts of peptide drugs
The key factors for the success of peptide drugs are the effectiveness, specificity and safety of the mode of action of the peptide [3]. The rapid clearance of peptides from the body means that they do not accumulate in tissues and are relatively less toxic to the human body [4]. However, the limitations of peptide drugs are as obvious as their advantages.
Since peptide drugs are easily cleared from the serum, this also results in low bioavailability of peptide drugs. Furthermore, peptides generally have poor cell membrane permeability, which limits their use in targeting intracellular targets. Therefore, the development of peptide therapeutics has mainly focused on extracellular targets. Moreover, because they cannot penetrate the intestinal mucosa and need to be administered subcutaneously or intravenously, the convenience and compliance of peptide drugs in actual treatment are reduced [4].
Improving the bioavailability and efficacy of peptide drugs is also a popular research area. There have also been advances in universal and reproducible oral administration, as well as intracellular delivery of peptide drugs [4]. Cyclic peptides, a category of peptide drugs, are an emerging form of drugs designed to solve problems.
Cyclotides—a new form of peptide drugs
Cyclic peptides (including cyclodeposition peptides and bicyclic peptides) have many favorable properties as therapeutic agents and research tools. Compared with linear peptides, cyclic peptides have better proteolytic resistance and structural stability.
First, cyclization of peptides reduces the conformational freedom, greatly enhancing their metabolic stability and binding affinity/specificity to target molecules. In general, cyclic peptides with smaller ring sizes (10 aa) are relatively resistant to proteolytic degradation. Second, medium-sized cyclic peptides (6-15 aa; most commonly used MW = 500-2000 Da) are usually 3-5 times larger than traditional small molecule drugs (MW 500) and can form more complex molecules with target proteins. Large surface area. Therefore, cyclic peptides have the ability to reproduce exceptional protein affinity and specificity, even for targets without any binding pocket. Third, cyclic peptides exhibit enormous structural diversity. Using only these 20 protein-derived amino acids, 208 different cyclic peptides can be generated. And its structural diversity can be further increased by adding non-protein source amino acids. Fourth, compared with proteins, cyclic peptides retain some properties of small molecules, such as stability, lower risk of immune response, synthesizability, and lower production costs [5].
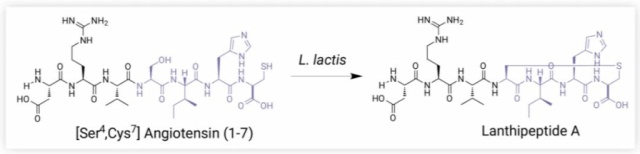
Figure 2. Angiotensin (1-7) is a heptapeptide that plays a key role in the renal angiotensin system, but its extremely short half-life limits its therapeutic potential. Moll uses a variant of Lactococcus lactis that produces the cyclic peptide Lanthipeptide A, which in rat studies showed an approximately 30-fold longer plasma half-life than Angiotensin (1-7) [4].
Currently, several cyclic peptides have become highly successful drugs, including vancomycin (antibacterial), daptomycin (antibacterial), cyclosporine A (transplantation immunosuppressant), and caspofenside (antifungal). Inspired by natural products, chemists have developed many methods to prepare cyclic peptides via N-to-C, side chain to side chain, or main chain to side chain cyclization. Some synthetic cyclic peptides, such as eptifibatide (used to treat heart disease), octreotide (a somatostatin mimetic used to treat acromegaly and diarrhea), cyclic RGD peptide, and linalotide Peptides have also been approved by the FDA for clinical or late-stage clinical trials [5].
Peptidomimetics – chemically synthesized peptide drugs
In terms of new drug strategies, in order to overcome the instability defects of peptides, in addition to modifying polypeptides to varying degrees like cyclic peptides, peptidomimetic compounds are also another reasonable means.
Peptidomimetic compounds are a class of compounds whose pharmacophore simulates natural peptides or proteins in three-dimensional space and retains the ability to interact with biological targets and produce the same biological effects [6]. The difference is that peptoids avoid the inherent defects of natural polypeptides and improve biological activity and stability.
Many non-peptide mimetics of bioactive peptides have been reported in the literature. αvβ3-integrin inhibitor SB223245 (Figure 3-1), γ-turn analog scaffold centered on 1-4 benzodiazepines, TRH (pGlu-His-Pro-NH2) analog (Figure 3-2), Contains a cis-1,3,5-trisubstituted cyclohexane scaffold, a glucose-derived non-peptide mimetic somatostatin (Figure 3-3), and c2 symmetric cyclic urea as an HIV protease inhibitor (Figure 3-4), These are outstanding examples of non-peptide peptidomimetics with high biological activity and increased enzyme stability [1].
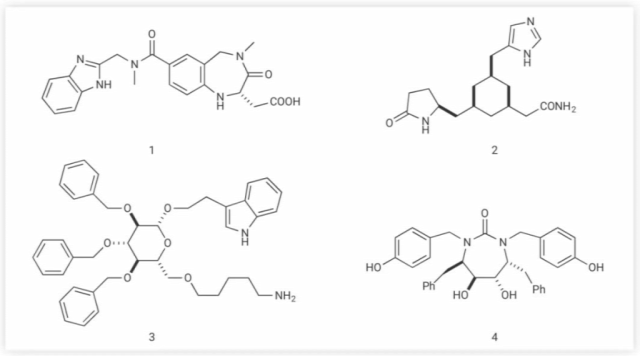
Figure 3. Chemical formulas of some peptidomimetics[1]
(1) SB223245; (2) TRH (pGlu-His-Pro-NH2) analog; (3) Glucose-derived non-peptide mimetic somatostatin; (4) c2 symmetric cyclic urea
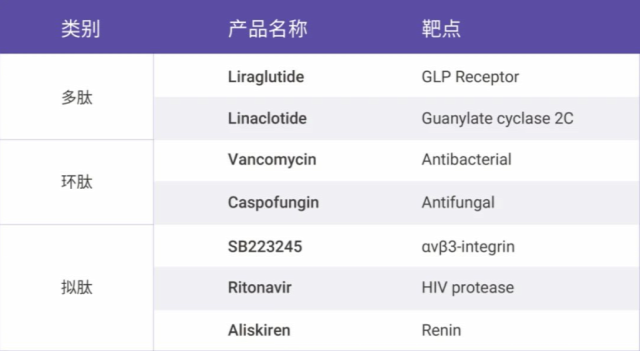 Table 1. Classic peptide drugs and their target
Table 1. Classic peptide drugs and their target
Peptide drugs, whether isolated from the innate immunity of various species (including mammals, amphibians, fish, insects, plants and bacteria), or designed based on structure-activity relationship research, serve as a new structural drug , all have great potential [7]. Here we introduce a high-throughput method that can quickly identify and find suitable drugs – constructing a peptide library.
The construction and use of peptide libraries generally involves gathering all the required peptides, uniformly culturing the samples, and then conducting high-throughput detection at the cellular or molecular level. For example, B. Guixer et al. determined the N-terminal and C-terminal forms by selecting the amino acids with specific functions they needed, and used the mixing-splitting method to synthesize a peptide library composed of different arrangements of the selected amino acids. Then, they conducted experiments simulating the blood-brain barrier to screen peptides that could pass through the peptide library, and finally obtained peptides (shuttle peptides) that had the ability to cross the in vitro blood-brain barrier model (Figure 4)[8].
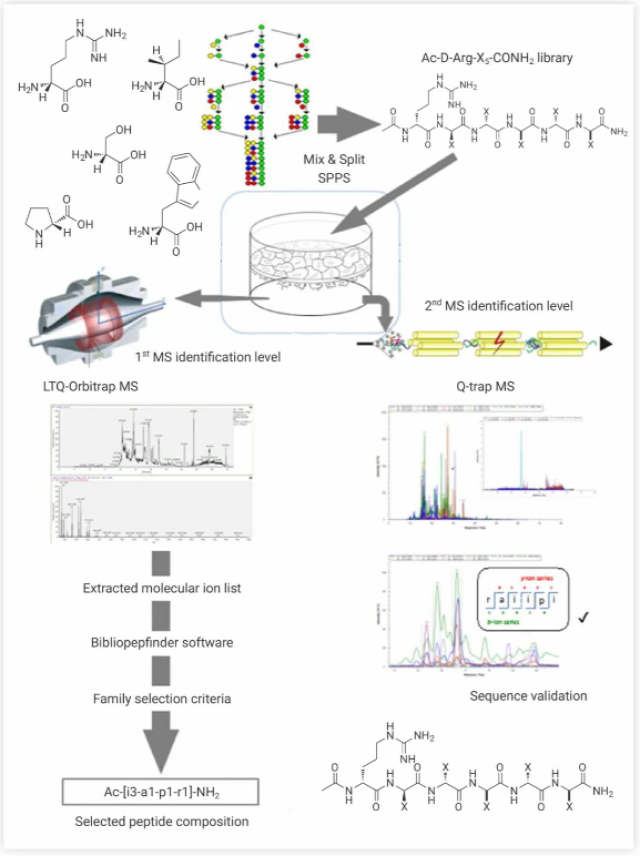
Figure 4. Overall scheme of mass spectrometry-based high-throughput screening method[8]
(1) Library synthesis using the mixed fraction method on SPPS (solid-phase polypeptide synthesis); (2) Library analysis in an in vitro cell-based blood-brain barrier model; (3) Peptide identification using mass spectrometry technology to monitor specific amino acid sequences transformation.
Currently, there are nearly a hundred peptide drugs on the global market, and research on new peptide therapeutic drugs continues at a steady pace, with more than 100 peptides in the clinical development stage and another 400-600 peptides in the preclinical research stage [2 ]. The utilization of peptides as therapeutics has evolved over time and continues to evolve as drug development and treatment paradigms change.
references
- Luca Gentilucci. Chemical modifications designed to improve peptide stability: incorporation of non-natural amino acids, pseudo-peptide bonds, and cyclization. Curr Pharm Des. 2010;16(28):3185-203.
- Markus Muttenthaler. Trends in peptide drug discovery. Nat Rev Drug Discov. 2021 Apr;20(4):309-325.
- Keld Fosgerau. Peptide therapeutics: current status and future directions. Drug Discov Today. 2015 Jan;20(1):122-8.
- Antoine Henninot. The Current State of Peptide Drug Discovery: Back to the Future?. J Med Chem. 2018 Feb 22;61(4):1382-1414.
- Patrick G Dougherty. Understanding Cell Penetration of Cyclic Peptides. Chem Rev. 2019 Sep 11;119(17):10241-10287. Epub 2019 May 14.
- Josef Vagner. Peptidomimetics, a synthetic tool of drug discovery. Curr Opin Chem Biol. 2008 Jun; 12(3): 292–296.
- Sylvie E Blondelle. Optimization and high-throughput screening of antimicrobial peptides. Curr Pharm Des. 2010;16(28):3204-11.
- B Guixer. Chemically synthesized peptide libraries as a new source of BBB shuttles. Use of mass spectrometry for peptide identification. J Pept Sci. 2016 Sep;22(9):577-91.


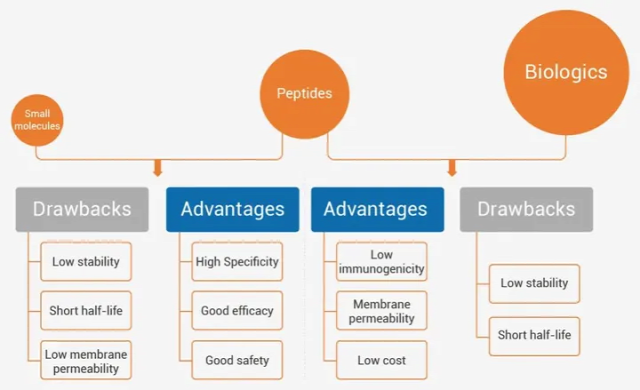
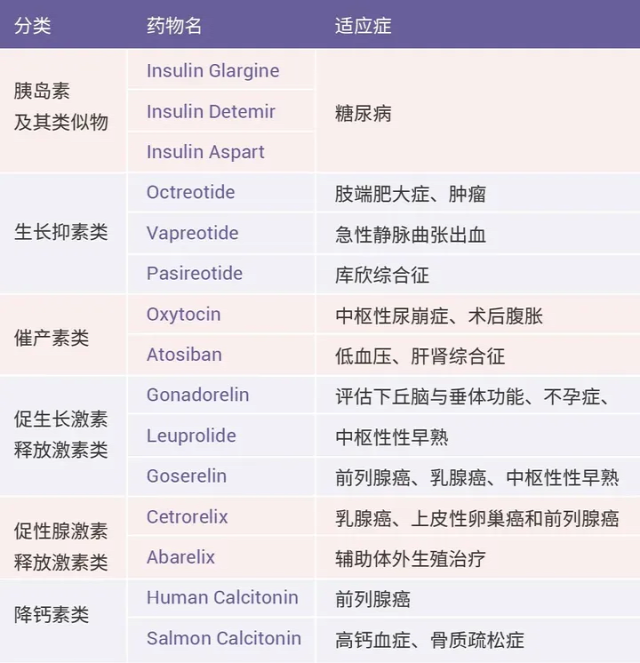



 Table 1. Classic peptide drugs and their target
Table 1. Classic peptide drugs and their target