Part1 Financing/BD Event
December 2023
- Kintide Pharmaceuticals completed US$8 million in seed round financing to advance a highly integrated computational design-automated synthetic peptide drug discovery platform
- Roche acquires Carmot Therapeutics for over US$3 billion, entering the GLP-1 competition
- Fractyl Health said in a regulatory filing that it plans to conduct an IPO in 2024. The company is committed to developing innovative treatments for type 2 diabetes and obesity, and the funds raised will be used to advance the development of its pipeline, including a gene therapy that expresses a GLP-1 receptor agonist in the pancreas.
- Amide Technologies announced that it has begun commercializing its novel peptide manufacturing platform and has completed in financing, including a Series A+ round led by Engine Ventures with participation from Forcefield Venture Fund. It previously raised a $3.55Mn Series A round led by Engine Ventures and a $5.45Mn Seed round led by Biological Engineering Ventures.
- Alkermes plc announced that it has entered into a definitive agreement to sell its development and manufacturing facility in Athlone, Ireland, to Novo Nordisk. Under the terms of the agreement, upon completion of the transaction, Alkermes will be entitled to a one-time cash payment of for the facility and associated assets, subject to customary adjustments in accordance with the agreement.
November 2023
- Zhifei Biotech plans to acquire 100% equity of Chongqing Chenan Biopharmaceutical Co., Ltd. in cash to enter the GLP-1 field
- Chengdu Taihe Weiye Biotechnology Co., Ltd. completed nearly 300 million yuan in Series A+ financing to accelerate the company’s Fmoc amino acid production capacity construction
- Peptide Biotech completed nearly 200 million yuan in Series B+ financing to promote the development of GLP-1 products
- lmagine Pharma completed US$32.5 million in Series A financing to develop new peptide IMG-1 and other projects
September 2023
- PeptiDream enters into a new multi-target collaboration and licensing agreement with Genentech, a Roche company, to discover and develop novel macrocyclic peptide-radioisotope (peptide-RI) conjugates
- Elicio Therapeutics receives $2.6 million foundation grant to fund research into two therapeutic cancer peptide vaccines
- Shenzhen Tuosheng Biotechnology Co., Ltd. announced the completion of tens of millions of RMB Pre-A round of financing
August 2023
- PeptiGrowth Inc (PeptiGrowth) and Orizuru Therapeutics, Inc (OZTx) have signed a joint development agreement to create a new synthetic peptide to replace recombinant growth factors used in the production of regenerative medicine products
- HBC lmmunology completed US$900,000 in seed round financing to advance the research and development of peptide drugs
June 2023
- Sino Biopharmaceuticals and Hongyun Huaning (Hangzhou) Biopharmaceutical Co., Ltd. have reached a cooperation agreement to jointly develop the dual-target innovative weight loss drug GMA106. GMA106 is a dual-target drug mainly suitable for the treatment of obesity, non-alcoholic fatty liver disease and diabetes
May 2023
- Carmot Therapeutics announces raising $150 million in super Series E funding to advance its development of treatments for obesity and diabetes
- lronwood successfully acquires next-generation GLP-2 through acquisition of VectivBio for $1 billion
Annual Highlights of Blockbuster Products
December 2023
- Eli Lilly announced that its injectable Zepbound (Tirzepatide) is now available in U.S. pharmacies
- Lisata Therapeutics announced that it has completed enrollment in the Phase IIb ASCEND study of its novel drug LSTA1 for the treatment of metastatic pancreatic ductal adenocarcinoma (“mPDAC”), achieving a key milestone.
- Italian company Nerviano Medical Sciences (NMS) and Spanish pharmaceutical company Italfarmaco (ITF) jointly announced that ITF will use NMS’s proprietary linker-payload technology to develop a new PDC product candidate.
- Apellis presented post-hoc data for its bicyclic peptide drug EMPAVELI® (pegcetacoplan) at the ASH Annual Meeting that strengthen the efficacy of EMPAVELI (pegcetacoplan) in adults with paroxysmal nocturnal hemoglobinuria (PNH) for up to three years. years of long-term efficacy and safety.
November 2023
- Eli Lilly announced that its GIP/GLP-1 receptor dual agonist Zepbound (Tirzepatide) has been approved by the U.S. FDA to reduce weight and maintain weight stability in obese or overweight adult patients.
- Novo Nordisk released its results for Q3 and the first three quarters of 2023. Sales of semaglutide in the first three quarters have exceeded US$14.2 billion.
- Eli Lilly and Company released its financial report for the third quarter of 2023. Sales of Tirzepatide in the first three quarters have reached US$2.957 billion.
October 2023
- Novo Nordisk announced that the Phase III clinical study FLOW of semaglutide in patients with type 2 diabetes and chronic kidney disease with renal insufficiency was terminated early due to excellent efficacy.
August 2023
- Eli Lilly’s Tirzepatide Injection Submits Registration Application in China for Long-term Weight Management Indication in Adults
July 2023
- Eli Lilly announced the results of two phase 3 clinical trials on tilpotide. The results showed that the average weight loss effect was as high as 26.6%, making it the most powerful weight loss drug in history.
Peptide drugs approved for marketing
December 2023
- Tonghua Dongbao Pharmaceutical’s marketing application for biosimilar GLP-1 analog liraglutide injection filed under registration classification 3.3 has been approved
- Ascendis Pharma announced that the FDA has accepted the company’s resubmitted New Drug Application for palopegteriparatide (TransCon PTH) for the treatment of adult patients with hypoparathyroidism.
- The National Medical Insurance Administration officially announced the adjustment list of the 2023 version of the National Medical Insurance Drug Catalog. The new round of medical insurance catalog includes four popular GLP-1 products. These include Renhui Biotech’s benaglutide injection, Lilly’s dulaglutide injection, Hanson Pharmaceuticals’ polyethylene glycol loxenatide injection, Novo Nordisk’s semaglutide injection, etc.
September 2023
- BioLineRx announces FDA approval of Aphexda (motixafortide) combined with filgrastim (granulocyte colony-stimulating factor G-CSF) to mobilize hematopoietic stem cells into the peripheral blood as a stem cell mobilization (SCM) to promote autologous transplantation in patients with multiple myeloma at the time of transplantation
- The marketing application for new indications of Lilly’s dulaglutide injection (trade name: Duida) was approved
July 2023
- Renhui Biotech’s benaglutide has been approved for marketing as a new indication for weight loss. This is the first original new weight loss drug in China, and the injection frequency is three times a day.
- East China Medicine’s Liraglutide Injection is approved for marketing for obesity or overweight indications
June 2023
- Hansoh Pharmaceutical’s Class 1 new drug “Pemoxatide Injection” (trade name: Saint Relais) was approved for marketing. This medicine is suitable for adult non-dialysis patients who are not receiving erythropoiesis stimulating agents (ESA), and adult dialysis patients who are receiving short-acting erythropoietin (EPO).
May 2023
- Blue Earth Diagnostics’ radiohybrid prostate-specific membrane antigen (PSMA)-targeted PET imaging agent Posluma (flotufolastat F 18) injection (formerly known as 18F-rhPSMA-7.3) was approved by the FDA for marketing
March 2023
- Acadia Pharmaceuticals Announces FDA Approval of DAYBUE (trofinetide) for the Treatment of Rett Syndrome in Adult and Pediatric Patients Two Years and Older
- Cidara Therapeutics’ New Drug Application (NDA) for Rezafungin for the treatment of candidemia and invasive candidiasis was approved by the FDA
January 2023
- Radius Health’s TYMLOS (abapatide) received U.S. FDA approval for new indication (osteoporosis)


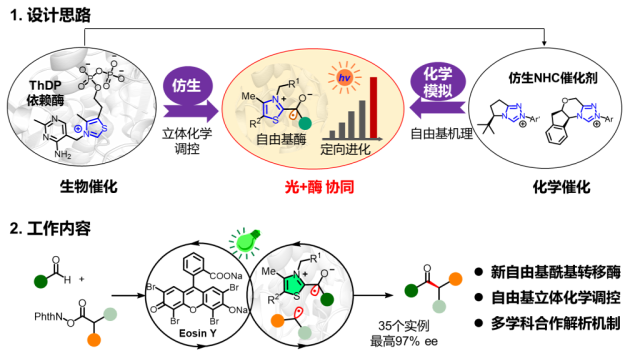
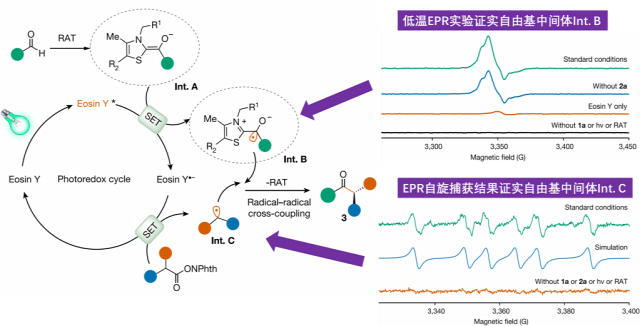
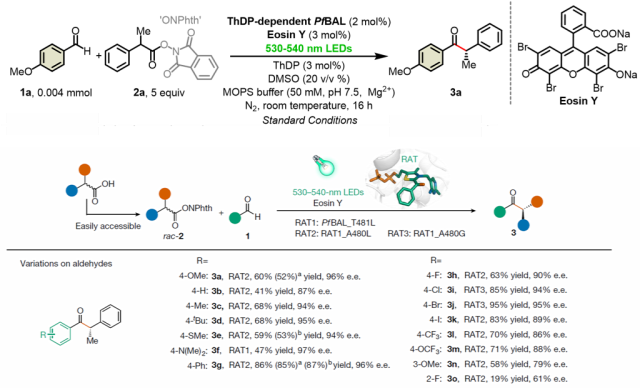 For the photoenzyme dual catalytic system, Professor Tian Changlin's team applied low-temperature (80K) electron paramagnetic resonance (EPR) experiments, capturing the ThDP-derived ketyl free radical (Int. B). Through EPR spin trapping experiments, they detected characteristic six-line splitting spectra in the standard reaction system, confirming it as an intermediate benzylic radical (Int. C) and the free radical product after addition with the capture agent. This provided direct evidence for unraveling the key to the new enzyme reactivity and the source of high stereochemical selectivity.
For the photoenzyme dual catalytic system, Professor Tian Changlin's team applied low-temperature (80K) electron paramagnetic resonance (EPR) experiments, capturing the ThDP-derived ketyl free radical (Int. B). Through EPR spin trapping experiments, they detected characteristic six-line splitting spectra in the standard reaction system, confirming it as an intermediate benzylic radical (Int. C) and the free radical product after addition with the capture agent. This provided direct evidence for unraveling the key to the new enzyme reactivity and the source of high stereochemical selectivity.